

These are called oxygen isotopes, and the most common ones are oxygen-16, oxygen-17, and oxygen-18. Depending on the number of neutrons that accompany them, different types of oxygen atoms are produced. So, how is it possible that different kinds of atoms exist -like the hydrogen, carbon and oxygen atoms that make up all living beings on the planet-, if they are made of the same subatomic particles? The answer is simple: the number of protons and neutrons that constitute the atom’s nucleus form the different elements.įor example, all of the oxygen atoms in the universe have 8 protons. The smallest of the subatomic particles is the electron, with a mass of 9,11×10^-31kg.Īll protons are the same, as are neutrons and electrons. Furthermore, protons have a mass of 1,67×10^-27kg, very similar to that of neutrons (1,675×10^-27kg). Neutrons, as the name implies, are electrically neutral. These are called subatomic particles, and each one has their own characteristics, such as electric charge and mass.įor example, all protons are positively charged, while all electrons are negatively charged. These are very small entities which are, in turn, made of even smaller particles: protons, neutrons and electrons. What is the molar massĪlmost all matter on Earth is made of atoms. For question or remarks please contact us.Where m is the mass, n the number of moles and M the resulting molar mass. The molecular weight of elements refers to periodic table of elements.īOC Sciences cannot be held responsible for errors in the calculation, the program itself or the explanation. Note: N n means the number of relative element. MW= N 1xMW 1+N 2xMW 2+N 3xMW 3+…+N nxMW n
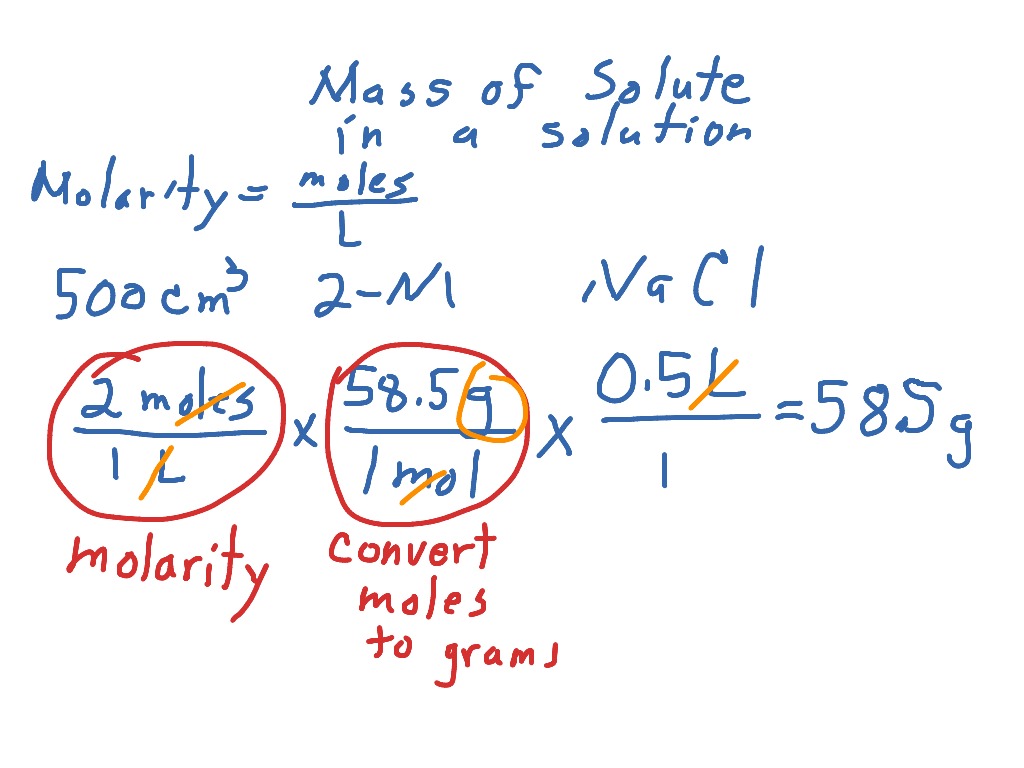
1 mol consists of exactly 6.02214076 * 10 23 molecules.

Molar mass (molar weight) is the mass of one mole of a substance and is expressed in g/mol. (1 u is equal to 1/12 the mass of one atom of carbon-12) Molecular mass (molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). a pollution of 1 gram of benzene in a certain amount of water converts to NA/78.11≈ 7.7098 × 1021 molecules polluting that water! Using the above calculator you could find that e.g. Or 1 mole of a substance will contain Avogadro's number of that substance. The term "mole" is defined in that one mole of a substance with a molecular (or atomic) mass of one, will have a mass of 1 gram.

Avogadro's number (N A) or Avogadro's constant (6.0221 x 10 23) is also important in this field. It is defined to be 1/12 of the mass of one atom of carbon-12 and in older works is also abbreviated as "amu". In related terms, another unit of mass often used is Dalton (Da) or unified atomic mass unit (u) when describing atomic masses and molecular masses. Molecular mass or molar mass are used in stoichiometry calculations in chemistry. To calculate molar mass of a chemical compound, please enter its chemical formula and click 'Calculate'.ĭefinitions of molecular mass, molecular weight, molar mass and molar weight: Instructions to calculate molar mass (molecular weight) of a chemical compound:


 0 kommentar(er)
0 kommentar(er)
